ARISTA™ COVID-19 Antigen Rapid Test
QUICKER AND MORE CONVENIENT TESTING THAN EVER BEFORE
The ARISTA™ COVID-19 Antigen Rapid Test is rolling out for self-test using the same reliable technology as our professional use product. That means you can now test for COVID-19 infection in the safety of your own home and get your result in 15 minutes. Super convenient with no line-up or travel time. And paired with the Viva Life App, you have the option to securely store all your records on your phone and generate a digital certificate whenever required. It’s all in your hands.
- Either you or a healthcare worker can collect your sample using the swab.
- Use the swab to mix the simple in the tube with diluent.
- Add the mixed specimen to the test cassette.
- Wait 15 minutes, then read your result.
- Generate a digital certificate and store your record using the Viva Life App.
HIGHLY SCALABLE FOR SAFER SCHOOLS, WORKPLACES, AND OTHER SPACES
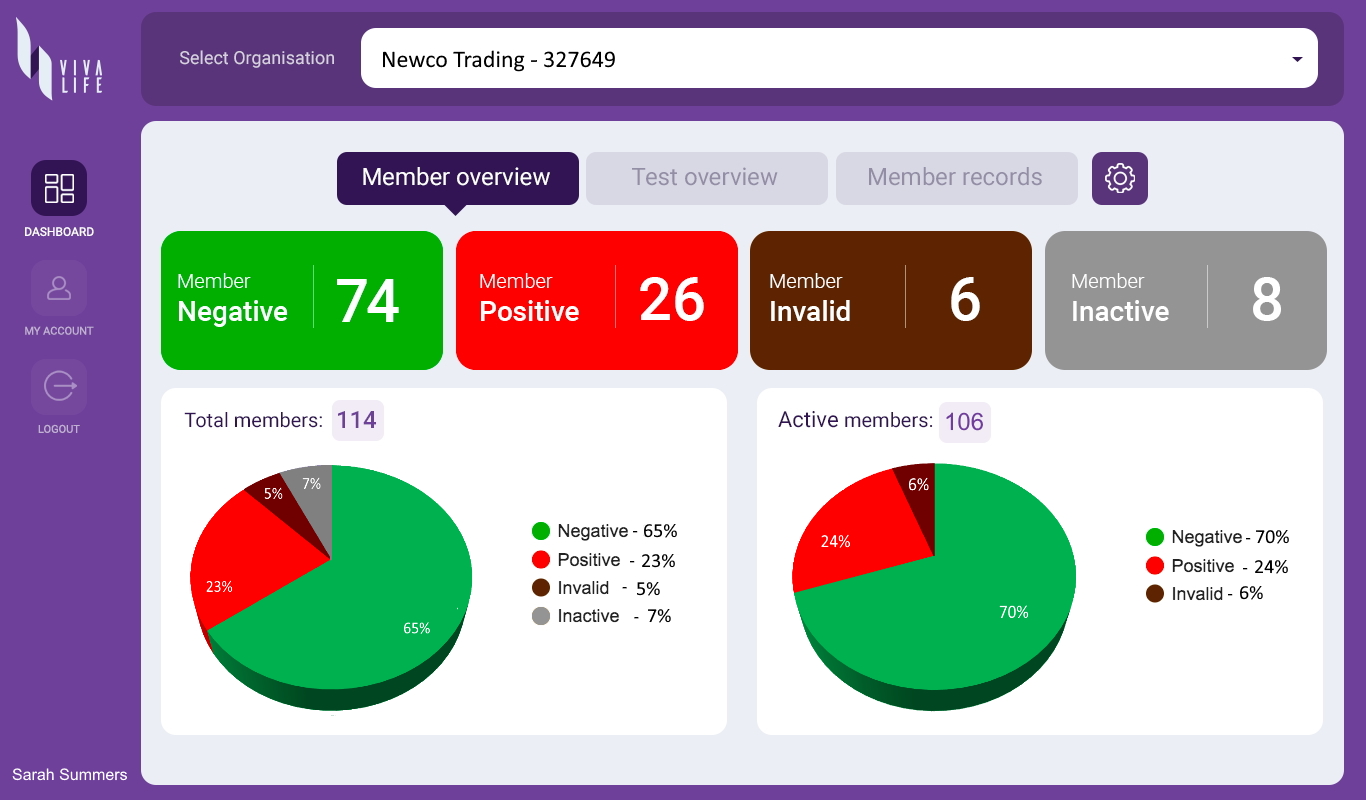
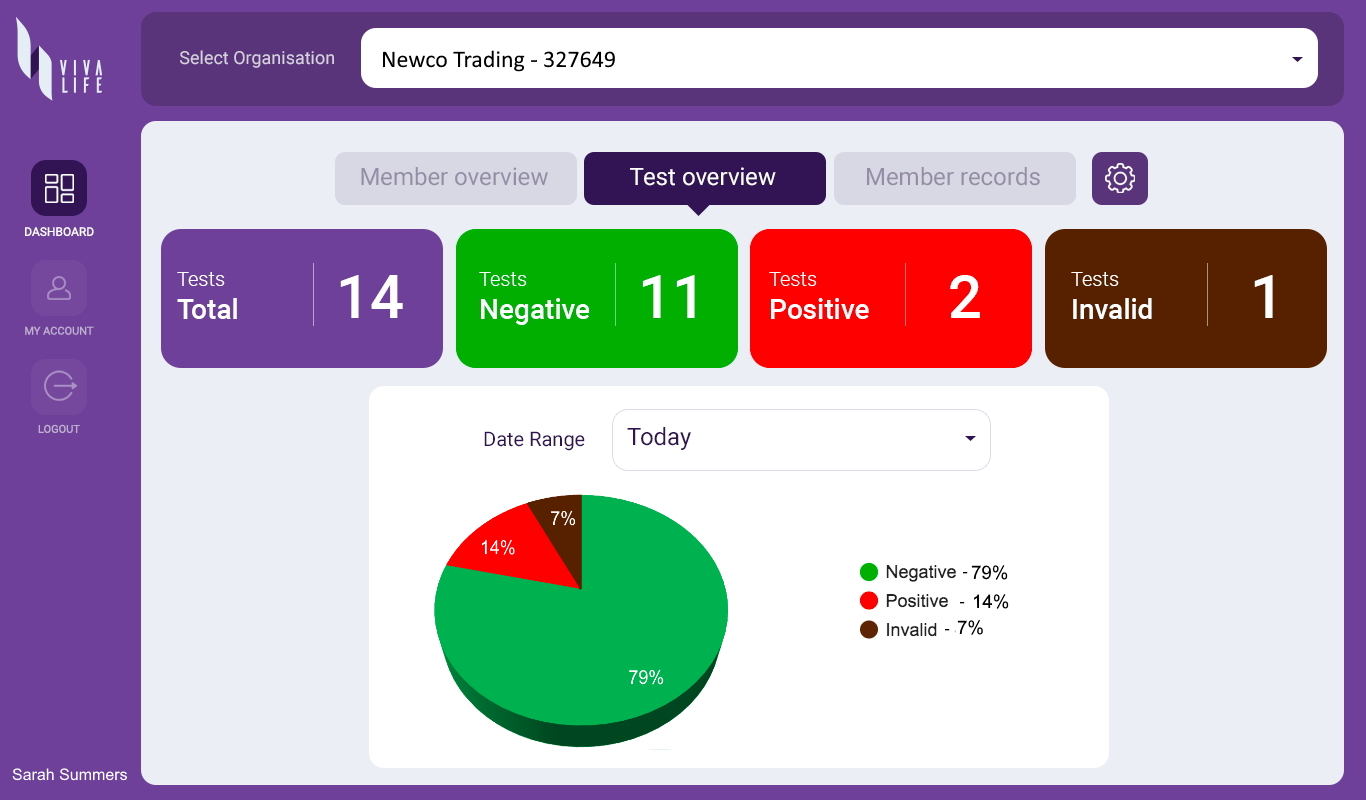
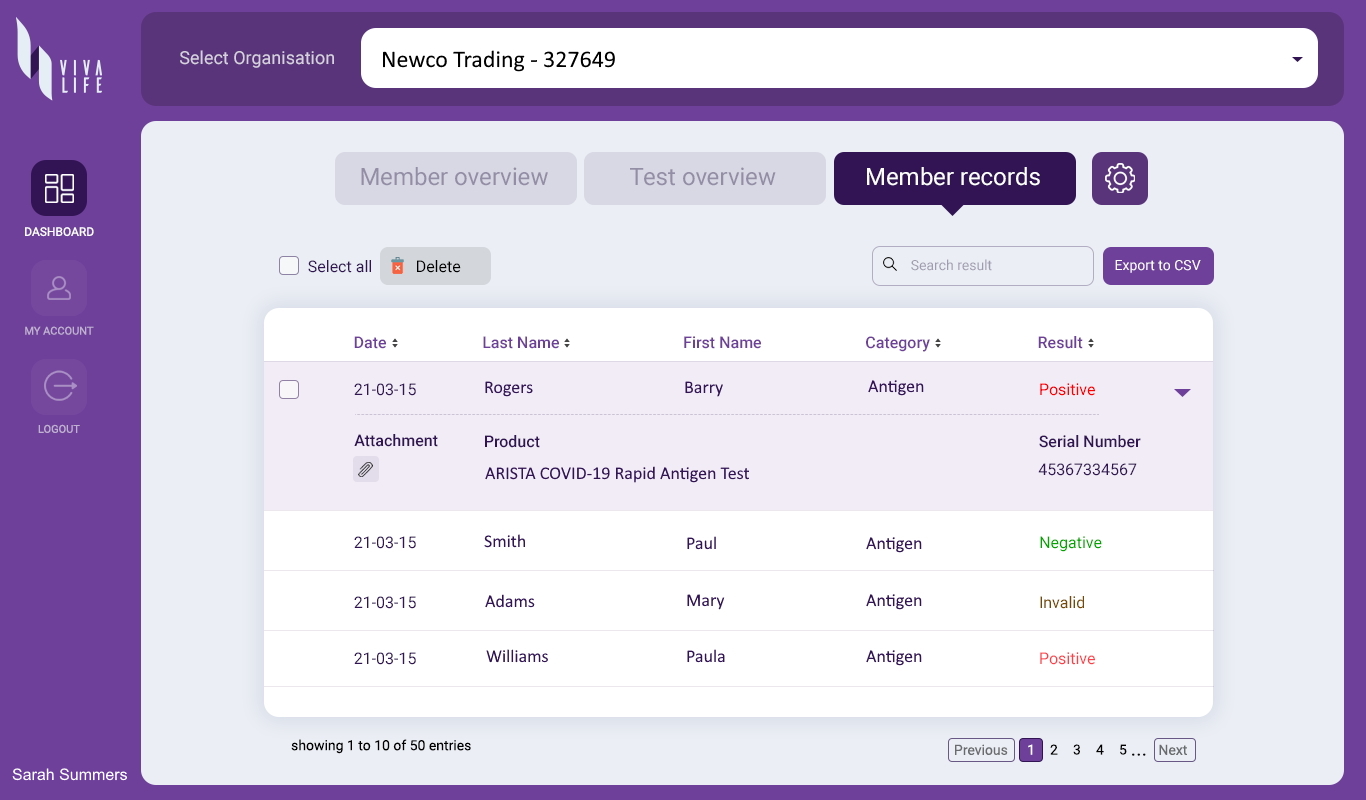
Even as vaccines are being made available, viral testing is fundamental to facilitating a safer return to everyday life. Our organizational testing program includes the Viva Life Enterprise Account – a web-based enterprise solution that enables individual Viva Life App users to sync their test results to an organization dashboard that they are associated with – such as their workplace or school. The organization receives instant data feedback on the health status of their people, allowing them to implement timely measures and provide support where necessary – creating a safer environment for everyone.
AVAILABLE FOR PROFESSIONAL USE AND SELF-TEST USE
The ARISTA™ COVID-19 Antigen Rapid Test is available for professional use at point-of-care by healthcare providers or personnel trained in any laboratory and non-laboratory environment that meets the requirements; and for self-test use. Both products offer the same reliable technology but differ somewhat in the sample specimen collection procedure. Nasopharyngeal and oropharyngeal specimen collection is only available at point-of-care settings, while anterior nasal and deep throat sputum specimen collection is available for self-test. In all cases, the patient can upload their results to the companion Viva Life App.
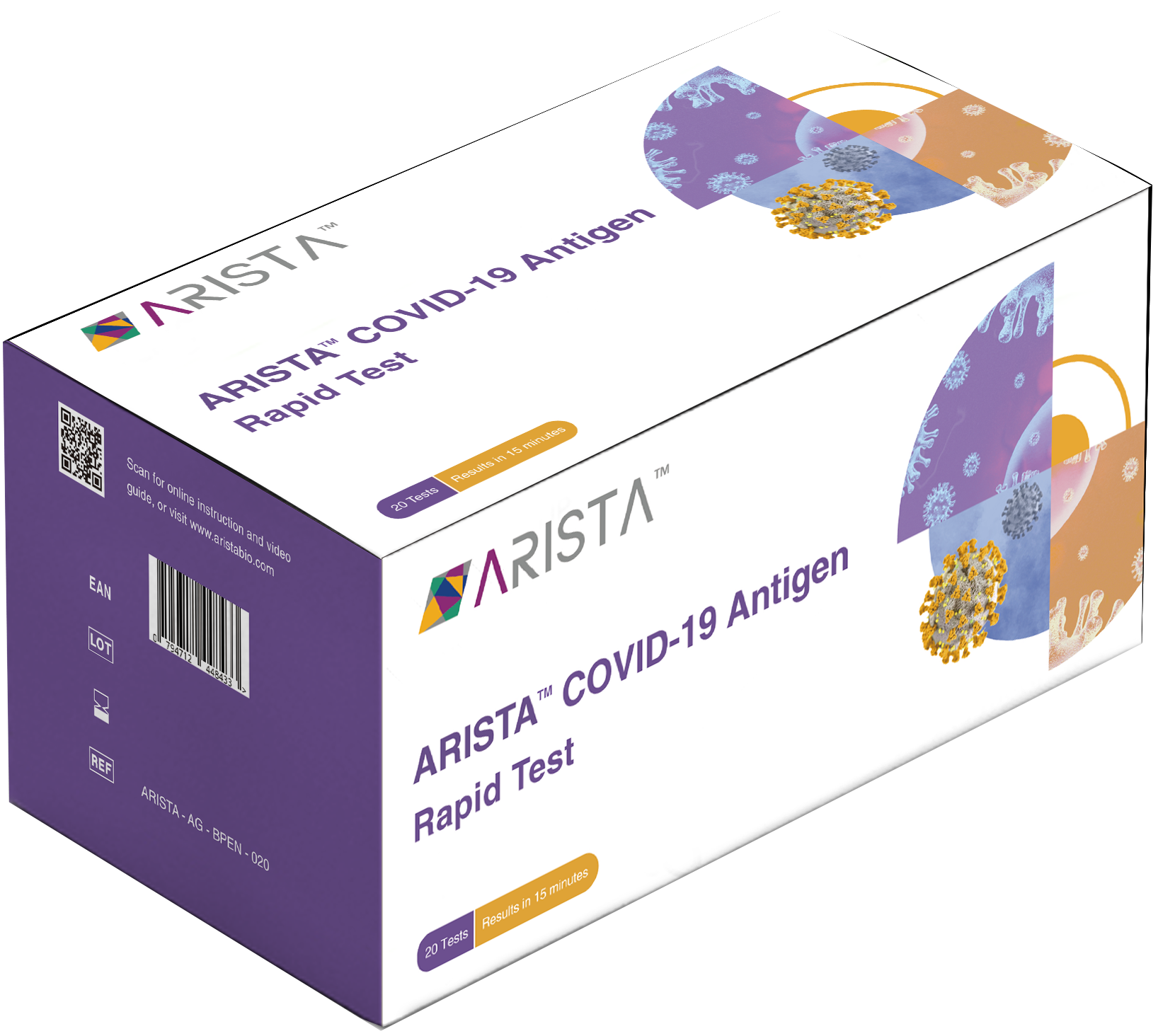
The ARISTA™ COVID-19 Antigen Rapid Test is a lateral flow immunoassay intended for the qualitative in vitro detection of SARS-CoV-2 virus nucleocapsid (N)-protein antigen in humans. The test is intended as an aid in identifying COVID-19 infection and is available for professional use at point-of-care by healthcare providers or personnel trained in any laboratory and non-laboratory environment that meets the requirements, and for self-test use in accordance with local regulations.
Performance studies conducted on the ARISTA™ COVID-19 Antigen Rapid Test for professional use with nasopharyngeal specimens indicated a sensitivity of 99.4% and a specificity of 100% with overall agreement to PCR of 99.82%.
Performance studies conducted on the ARISTA™ COVID-19 Antigen Rapid Test for self-test use with anterior nasal specimens indicated a sensitivity of 95.8% and a specificity of 100% with overall agreement to PCR of 98.55%.
Performance studies conducted on the ARISTA™ COVID-19 Antigen Rapid Test for self-test use with deep throat sputum specimens indicated a sensitivity of 97.9% and a specificity of 100.00% with overall agreement to PCR of 99.26%.
Performance studies conducted on the ARISTA™ COVID-19 Antigen Rapid Test for self-test use with saliva specimens indicated a sensitivity of 95.8% and a specificity of 100.00% with overall agreement to PCR of 98.55%.
The ARISTA™ COVID-19 Antigen Rapid Test complies with European In-Vitro Diagnostic Devices Directive 98/79/EC, and was issued HSA Provisional Authorisation (MDPA2020-180) for supply in Singapore and export from Singapore.





