Orawell® COVID-19 IgM/IgG Rapid Test
Intended use
Orawell® COVID-19 IgM/IgG Rapid Test is based on Lateral Flow Immunoassay (LFI) intended for qualitative detection of IgM and IgG antibodies to SARS-CoV-2 in human whole blood (fingertip), serum or plasma. This test is for in vitro diagnostic use only. It is intended for use as an aid in
identifying individuals with an adaptive immune response to SARS-CoV-2,
indicating recent or prior infection or a response to the COVID-19 vaccine. At
this time, it is unknown how long antibodies persist after infection or
vaccination and how long will protective immunity last.
Storage conditions
- The product should be stored at 2-30°C, away from direct sunlight
- The test cassette must remain in the sealed foil pack until use. Do not freeze or refrigerate
Test kit components

Test Cassette

Diluent

Lancet
Micropipette
Alcohol swab
Sample collection
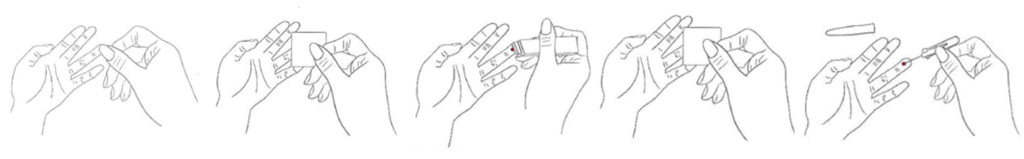
- 1. Wash and dry your hands well
- 2. Choose the fingertip where you will be obtaining blood, massage it and wipe with alcohol swab
- 3.Break the safety seal on the lancet. Prick slightly on the side of the fingertip, 2-3 mm deep, and pull the needle out immediately
- 4. After drop flows naturally, wipe away the first drop with another alcohol swab
- 5.Using the micropipette draw up the blood from the fingertip to the marked line on the micropipette and then press wound with alcohol swab to stop bleeding; if the blood flow is not enough, press the finger (from the palm towards the finger) slightly to make blood flow out
Conducting the test
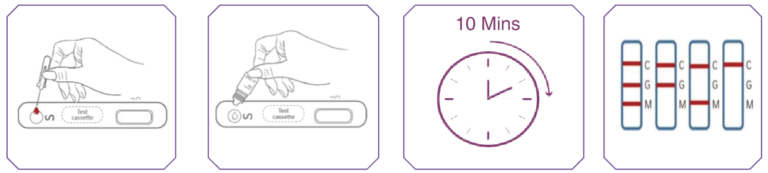
- 6. Bring the test kit components to room temperature
- 7. Place the test cassette on a level surface and squeeze 2 drops of whole blood from micropipette to the “S” well of the test cassette
- 8.Add 2 drops of the diluent to the “S” well of the test cassette
- 9. Wait 10 to 15 minutes. Ensure no disturbances to the test cassette
- 10.Read the test results
- 11. Dispose all materials in a plastic bag and wash your hands thoroughly
Understanding the test result
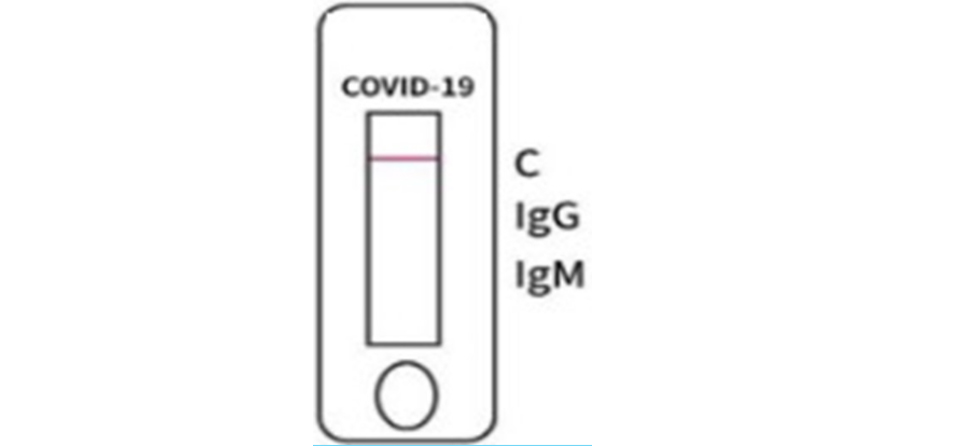
NEGATIVE
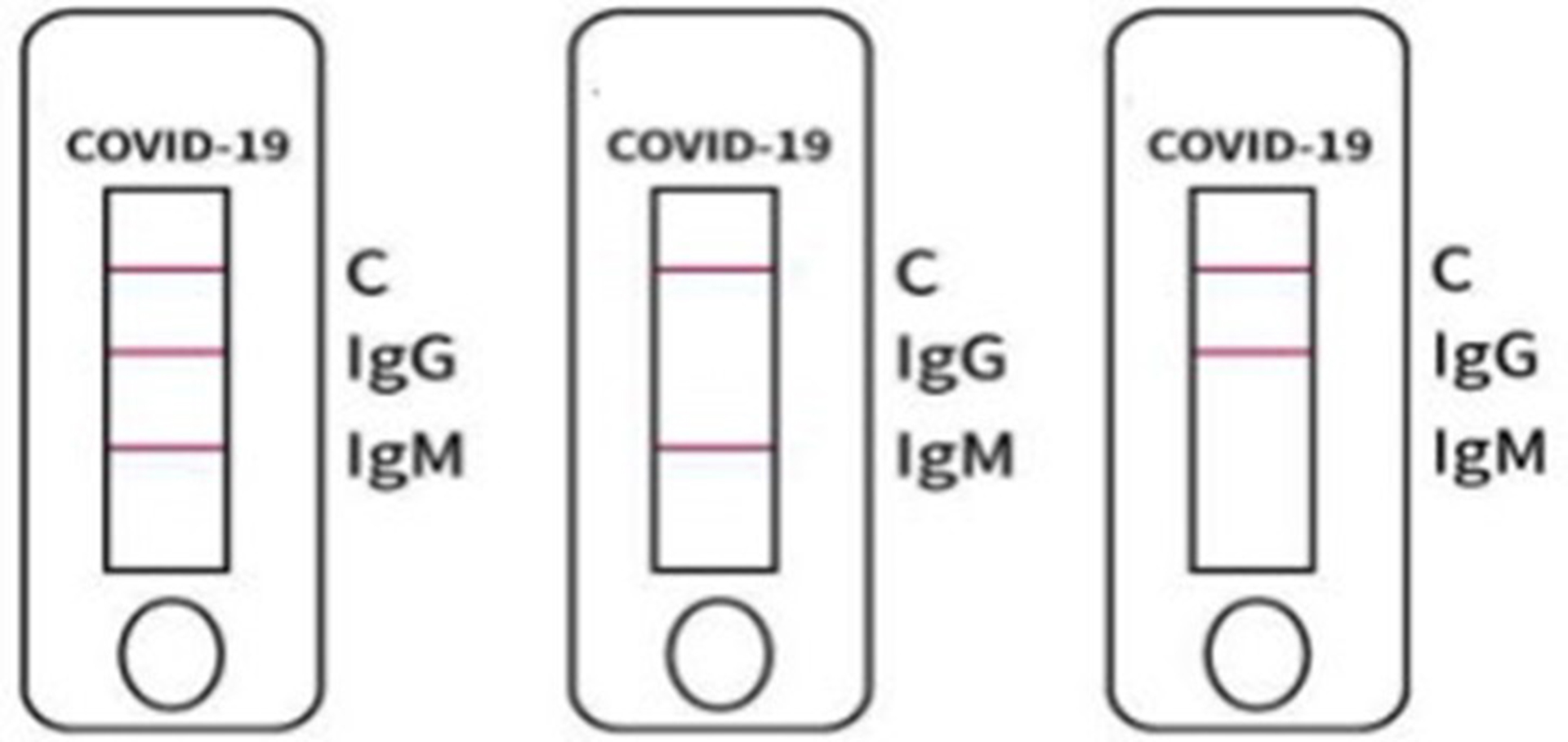
POSITIVE
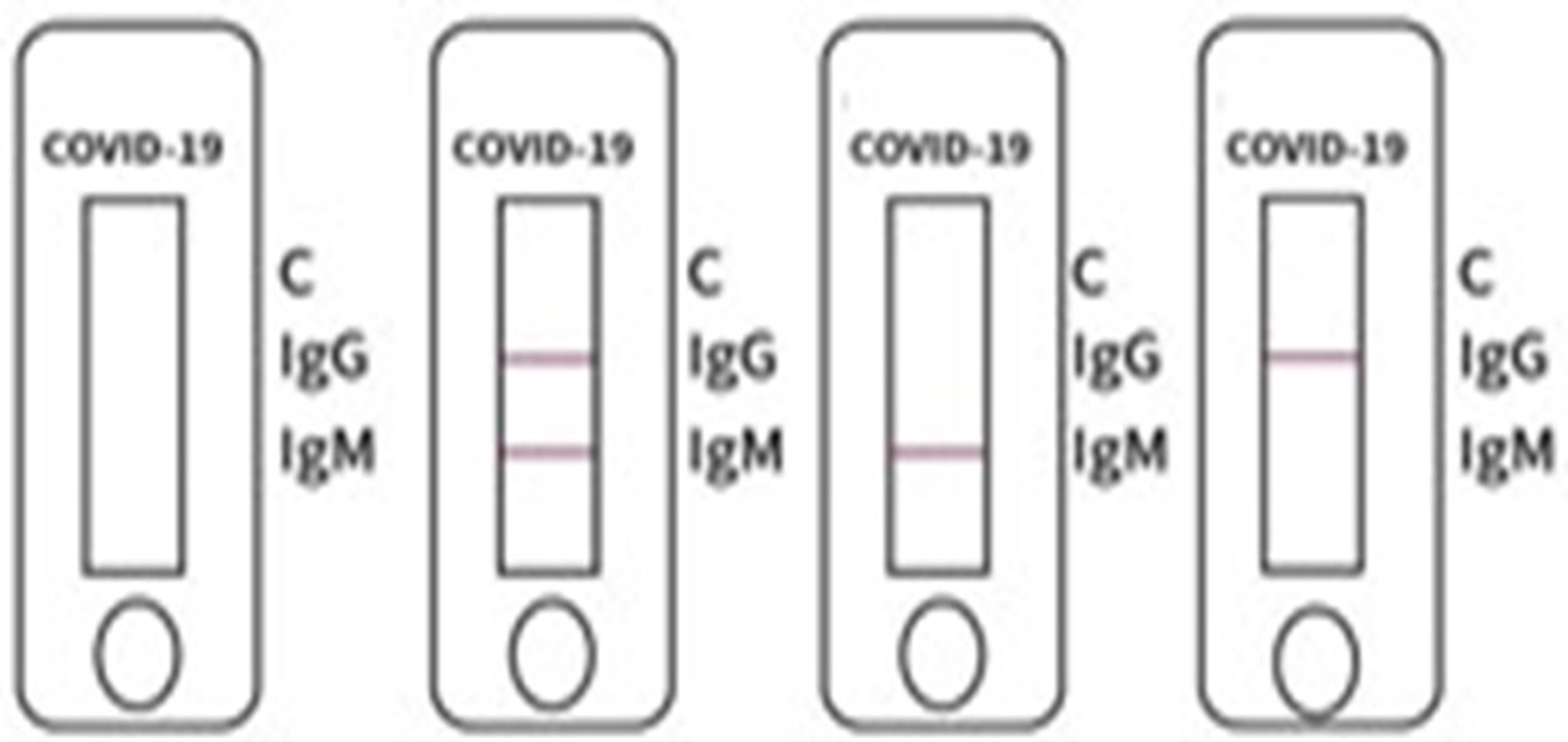
INVALID RESULT
1. Negative Result: if the quality control mark “C” displays a colored line, and the test mark “IgG” or “IgM” is blank, then the test result is negative2. Positive result: if the quality control mark “C” and the test mark “IgG” both display colored lines, but the test mark “IgM” is blank, then the test result is positive for COVID-19 IgG antibodies
3. Positive result: if the quality control mark “C” and the test mark “IgM” both display colored lines, but the test mark “IgG” is blank, then the test result is positive for COVID-19 IgM antibodies
4. Positive result: if the quality control mark “C” and both the test mark “IgM” and “IgG” both display colored lines, then the test result is positive for both COVID-19 IgM and IgG antibodies
5. Invalid result: if the quality control mark “C” is blank, no matter whether the test mark for “IgM’ or “IgG” is colored or not, the test result is invalid



