ARISTA® COVID-19 Neutralizing Antibody Test
MEASURING IMMUNE RESPONSE AFTER COVID-19 VACCINATION
The ARISTA® COVID-19 Neutralizing Antibody Test is able to semi-quantitively measure your body’s immune response to the SARS-CoV-2 virus following vaccination. The test can monitor the increase or decrease in the presence of neutralizing antibodies in blood, serum, or plasma samples – and yield results in 15 minutes.
Paired with the Viva Life App, users have the option to securely store their test records on their phone and generate a digital certificate whenever required. And compatible Viva Life Enterprise Account, users can further sync their test results to an organization dashboard that they are associated with – such as their workplace or school, allowing the organization to receive instant data feedback on the health status of their people, and enabling them to implement timely measures in creating a safer environment for everyone.
NEUTRALIZING ANTIBODIES ARE A KEY PREDICTOR OF IMMUNE RESPONSE
Neutralizing antibodies are produced following an infection or a vaccination. They are a specific sub-type of antibody that binds to the virus and acts like a key by locking the receptor and preventing the SARS-CoV-2 virus spike protein from entering the target cells thereby immediately neutralizing the harmful effect of the virus. While other antibodies attempt to work in the same way, some are known as non-neutralizing antibodies (or binding antibodies) because they do not bind to the precise region required to lock the receptor. They do however still have a role to play in generating an immune response by marking the virus as a target for recruited immune cells to attack and destroy.
Currently, most COVID-19 antibody tests are indicated to detect the broader binding IgM/IgG antibodies. The ARISTA® COVID-19 Neutralizing Antibody Test is however indicated to detect specific neutralizing antibodies. While a positive result from either test type is an indication of an immune response following infection or vaccination, the neutralizing antibody test may hold greater significance because of the neutralizing antibody’s immediate neutralizing effects on the virus. Furthermore, neutralizing antibody titers are a key measure being followed by vaccine researchers and regulators as a marker to assess the immunogenicity of COVID-19 vaccine candidates. Current data indicates that it takes between 7 to 21 days post-vaccination to develop neutralizing antibodies.
TRACKING YOUR IMMUNE RESPONSE
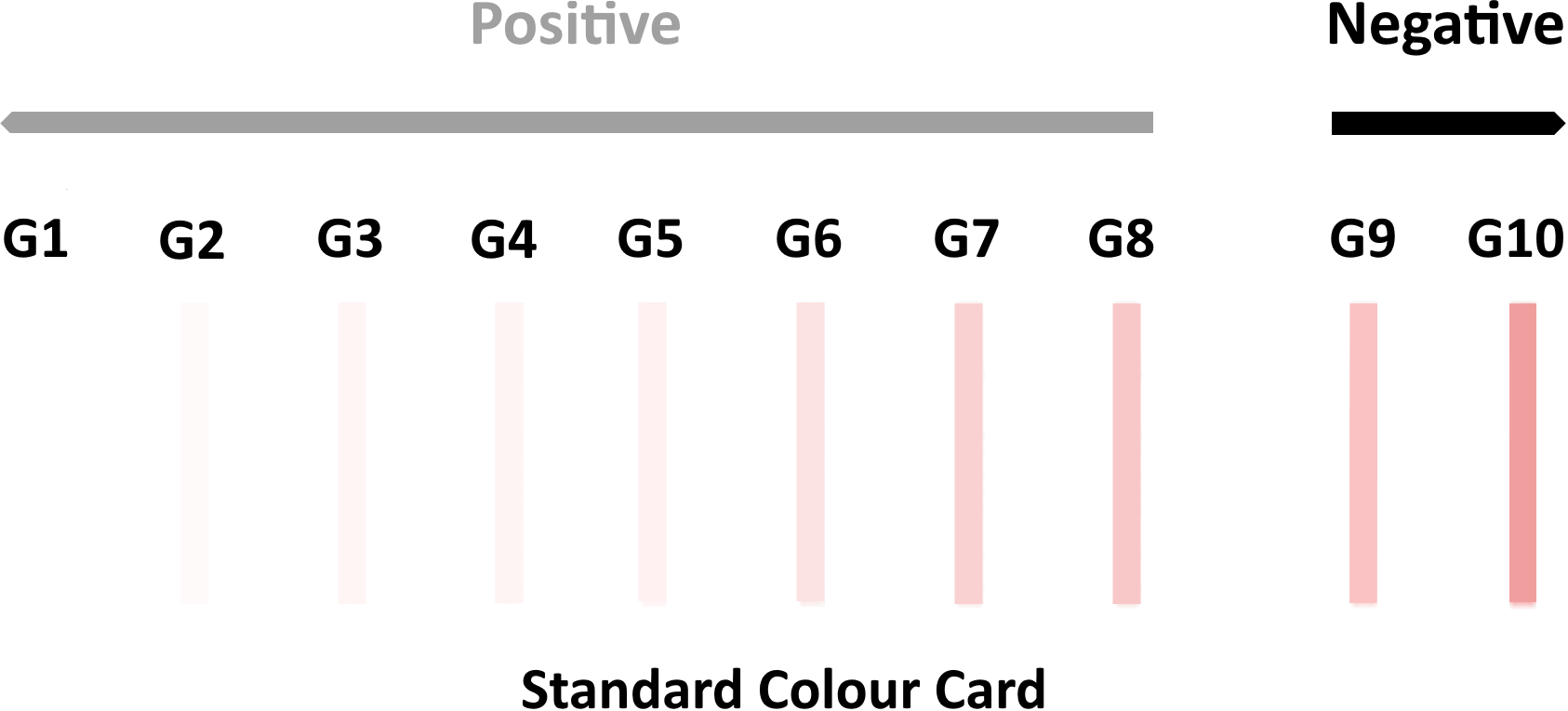
The ARISTA® COVID-19 Neutralizing Antibody Test is able to semi-quantitively measure the body’s immune response to the SARS-CoV-2 virus following vaccination using a variable colour intensity indicator on the test cassette to represent the concentration of neutralizing antibodies in the sample specimen. When implementing a test regime over a period of time, the change in intensity of the colour indicator will represent a relative increase or decrease in the presence of neutralizing antibodies in the individual.