news
2021-12-10
ARISTA Biotech Pte. Ltd evaluates Omicron and other COVID variants to ensure antigen test effectiveness
ARISTA COVID-19 Antigen Rapid Test is able to detect the emerging B.1.1.529 (Omicron) variant
Singapore, 9th December 2021 – ARISTA Biotech Pte Ltd (“ARISTA”) confirms that its ARISTA COVID-19 Antigen Rapid Test which tests for the presence of SARS-CoV-2 virus in clinical specimens by detecting the nucleocapsid protein (N-protein) in the core of the virus, is able to detect the emerging B.1.1.529 (Omicron) variant.
On 26th November 2021, the World Health Organization (WHO) announced and designated variant B.1.1529 named Omicron, a newly identified variant first identified in Botswana and South Africa, as a Variant of Concern. Early indication suggested this variant may have higher transmissibility and has already spread to over 30 countries in an accelerated manner.
Sequence study of the Omicron variant confirmed that it harbors more than 30 mutations across its genome with the majority of mutations in the Spike protein. The Omicron variant possesses three replacement and one deletion mutations in the N-protein compared to the original viral strain (Table 1).
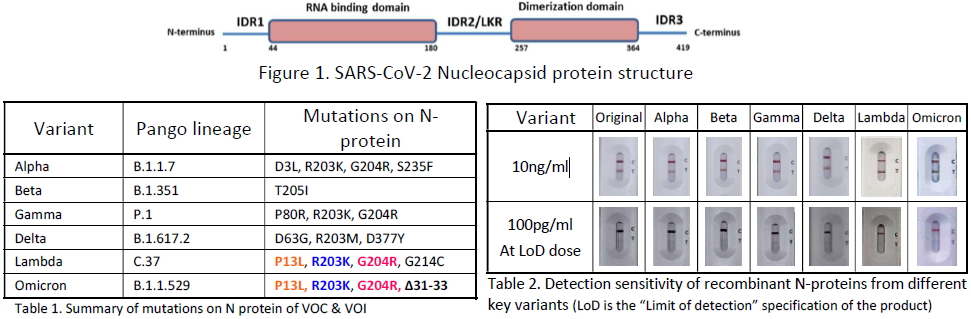
ARISTA has conducted studies using recombinant variant N-proteins to validate and confirm the ARISTA COVID-19 Antigen Rapid Test is able to detect over 400+ variants, including B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), B.1.617, B.1.617.2 (Delta), AY.1/AY.2/AY.3 (Delta Plus), B.1.617.3, C.37 (Lambda) and B.1.1.529 (Omicron) with similar sensitivities (Table 2).
Dr. Eric Tang, Chief Scientific Officer at ARISTA stated: “Our preliminary laboratory results indicate that the sensitivity of the ARISTA COVID-19 Antigen Test remains similar for all variants tested to date – including the Omicron variant. Further performance validation results and clinical studies on the Omicron variant are underway and will be made available in due course.”
