ARISTA™ COVID-19 Antigen Rapid Test
Self-test quick reference guide: Download
Important safety information
- 1. Thoroughly sanitize your hands with soap and water or hand sanitizer before conducting the test.
- 2.Only use the provided sterile swab, being careful avoid touching the sponge tip with your hands, or contacting it with any foreign surface when removing it from the peel-pack.
OPERATION GUIDE VIDEOS
ARISTA™ COVID-19 Antigen Rapid Test - Operation Guide for Nasal Sample
TEST KIT COMPONENTS

Disposable swab

Sputum Dish

Dropper cap

Tube with diluent

Test cassette
SAMPLE COLLECTION METHODS
Four methods of sample collection are described in this guide.
A. Anterior nares nasal swab specimen
For self-test collection or at point-of-care.
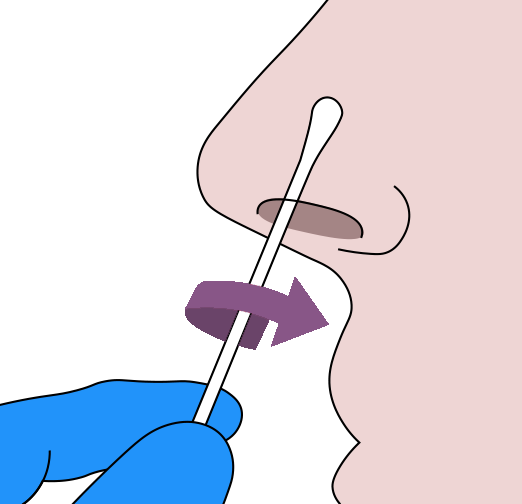
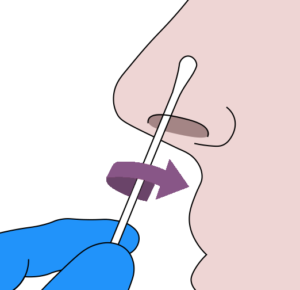
- 1. Thoroughly sanitize your hands.
- 2.Remove the sterile swab from the peel-pack being careful to avoid touching the sponge tip with your hands or contacting it with any foreign surface.
- 3.Insert the swab sponge tip 1.5cm (3/4 of an inch) into your nostril.
- 4.Slowly rotate the swab in a circular path against inside of your nostril at least 4 times for a total of 15 seconds.
- 5. Using the same swab, repeat steps 3-4 in the other nostril.
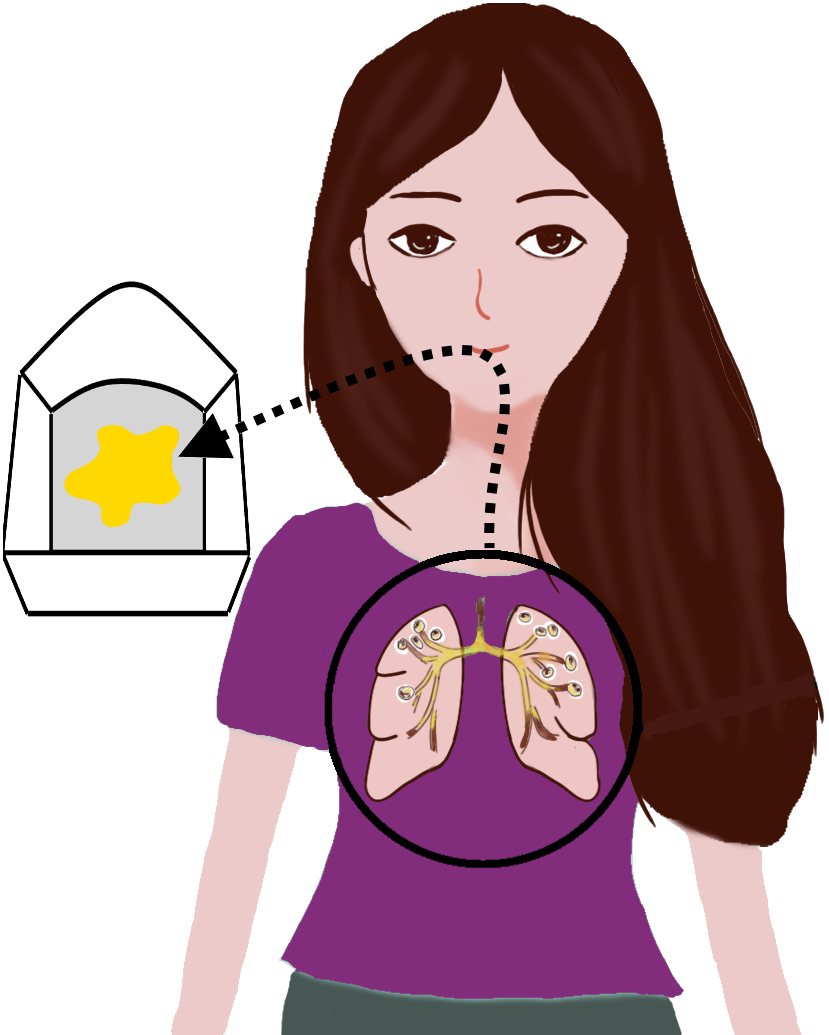
B. Deep throat sputum specimen

For self-test collection or at point-of-care.
- 1. Thoroughly sanitize your hands.
- 2.Inhale deeply and exhale forcefully through your mouth, repeat three times.
- 3.Cough deep from within your chest to produce sputum.
- 4. Spit your sputum (slight yellow/green color) into the sputum dish.
- 5.Remove the sterile swab from the wrapper being careful to avoid contacting the sponge tip with any foreign surface.
- 6.Fully cover the swab sponge tip with sputum by placing it in the sputum dish and applying a twisting motion.
C. Nasopharyngeal swab specimen
For collection at point-of-care.
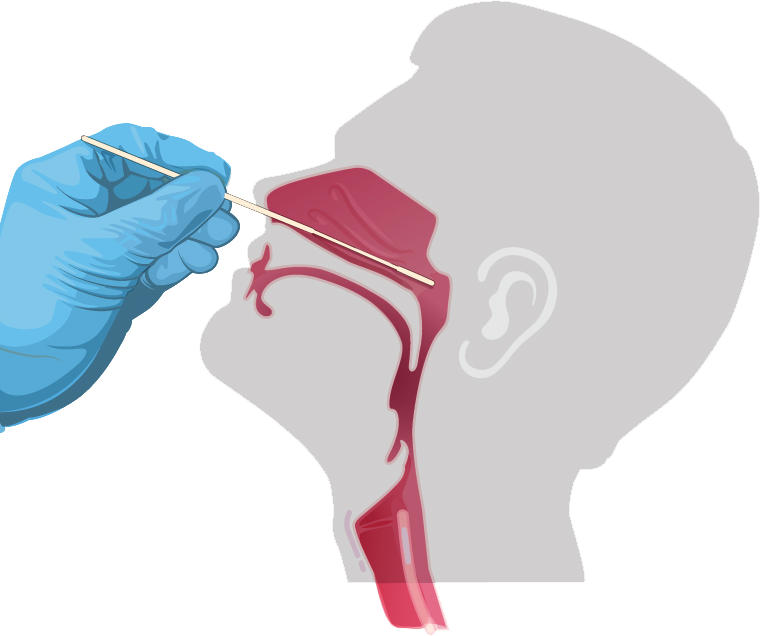
- 1. Thoroughly sanitize your hands.
- 2. Tilt the patient’s head back 70 degrees.
- 3. Remove the sterile swab from the peel-pack being careful to avoid contacting the sponge tip with any foreign surface.
- 4. Gently and slowly insert the swab sponge tip through the nostril parallel to the palate until resistance is encountered.
- 5. Gently rub and roll the swab, leaving it in place for several seconds to absorb secretions. If a deviated septum or blockage creates difficulty in obtaining the specimen from one nostril, use the same swab to obtain the specimen from the other nostril.
- 6. Slowly remove swab while rotating it.

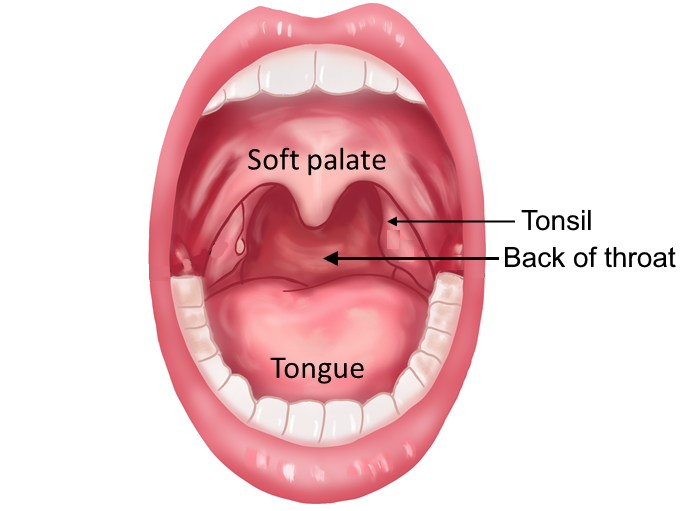
D. Oropharyngeal swab specimen

For collection at point-of-care.
- 1. Thoroughly sanitize your hands.
- 2. Remove the sterile swab from the peel-pack being careful to avoid contacting the sponge tip with any foreign surface.
- 3. Instruct the patient* to open their mouth and say “ahhh”.
- 4. Swab the back of the throat including the left and right tonsil area, taking care not to contact the sponge tip with any other part of the mouth.
* When swabbing children, it is advisable to use a tongue depressor for ease of application and additional safety.
INTERPRETING THE TEST RESULT
Negative test result
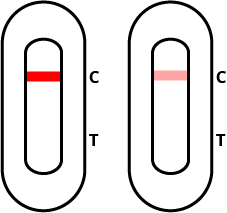
If the quality control “C” line is red (even if faint), and the test “T” line is blank, then the test result is negative and you are unlikely to have COVID-19.
Note: Negative results are presumptive should not be used as the sole basis for infection control decisions, particularly in the presence of clinical signs and symptoms consistent with COVID-19, or in those who have been in close contact with the virus. In these cases, it is recommended that the test is repeated after 1-2 days. In all cases, a definitive diagnosis must be made by a doctor and local health and safety control measures should always be adhered to.
Positive test result
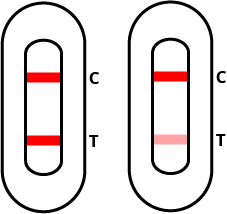
If both the quality control “C” line and the test “T” line is red even if faint) then the test result is positive and you are most likely infected with COVID-19.
Note: Positive results require strict adherence to your local public health authority’s guidelines for suspected infections – including confirmatory PCR screening, self-isolation, and reporting to the applicable authority.
Invalid test result

If the quality control “C” line is blank, then the test is considered invalid irrespective of any red band on the “T” band.Note: Invalid results are represented by a complete absence of a visible “C” line. In this case, carefully read the “Instructions for Use” and repeat the test. If a repeated test result is invalid, then contact your local product supplier.
Warning and precaution
- 1. The product should be stored at 3°-30°C in its original packaging, away from direct sunlight.
- 2. Follow the “Instructions for Use” for optimal performance of the test.
- 3. Do not use the product after the expiration date printed on the outer pack.
- 4. Follow standard precautions when handling specimens.
- 5. In event of spillage, clean with a suitable detergent.
- 6. Only use the swab provided, and do not swap out any of the components with other batches or products.
- 7. The diluent is not edible. Do not swallow.
- 8. The test should be performed at 15°-30°C (59°-86°F) as the test cassette is sensitive to humidity and heat. Only open the foil pack immediately before use and complete the test procedure within 30 minutes. Do not use if the foil pack is damaged or the test cassette has previously been exposed to environmental conditions beyond those specified.
- 9. Do not reuse any materials or components.
- 10. Dispose used materials according to local guidelines.