news
2022-07-15
ARISTA Biotech Confirms the Sensitivity of Rapid Antigen Test against Emerging Omicron Sub-Lineages BA.4 and BA.5
Performance was found to be similar to the detection sensitivity against the original virus.
Globally, the number of COVID-19 cases has increased for the fourth consecutive week, according to the World Health Organization (WHO) on 6th July 2022. The Omicron Variant of Concern (VOC) continues to be the dominant variant circulating, and it is proposed that two Omicron sub-lineages, BA.4 and BA.5, are responsible for the recent escalation of COVID-19 cases. Early indication suggests it has higher transmissibility and a greater ability to bypass immunity from past COVID infection or vaccination.
While the mutation of Omicron and sub-lineages primarily occur in the spike (S) protein, there are eight mutations in BA.4 and seven in BA.5, of which six were seen before in the original Omicron (Table 1).
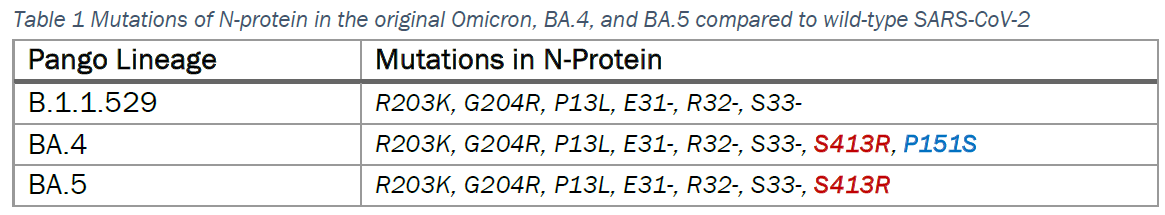
The ARISTA COVID-19 Antigen Rapid Test is designed to detect the N-protein of the virus. In all earlier studies using the N protein, inactivated virus, and clinical samples sequenced as the Omicron variant, the product’s performance has shown detection sensitivity similar to the original wild type. This was again confirmed in a recent study using recombinant N-proteins of the BA.4 and BA.5 sub-lineages of Omicron variant (Figure 1).

ARISTA will continue to conduct additional studies using clinical samples to affirm the clinical performance of the ARISTA™ COVID-19 Antigen Rapid Test in the detection of BA.4 and BA.5. The results of the studies and additional information will be publicly shared in due course.
For further information, contact us at info@aristabio.com